Water is a valuable resource with unique properties that allows it to be used in many different ways every day. To make sure it is safe, we measure the quality of water.
Let's take a look
An amazing 74 percent of the earth is covered by water. This water is continually moving around, through, and above the Earth as liquid water, ice, and water vapour. It is continually changing between these three forms. But did you know that the same water that existed on Earth millions of years ago is still here? Because the Earth is a "closed system", the Earth neither gains nor loses much matter, including water! Thanks to the water cycle, the same water is continually being recycled all around the globe.
Of all the freshwater on Earth, only about 0.3 percent is contained in rivers and lakes, but that's where most of the water we use in our everyday lives comes from. In fact, the majority of Earth's freshwater, about 69 percent, is locked up in glaciers and icecaps, mainly in Greenland and Antarctica. As for the rest of the water, the world's oceans make up about 97 percent of all water.
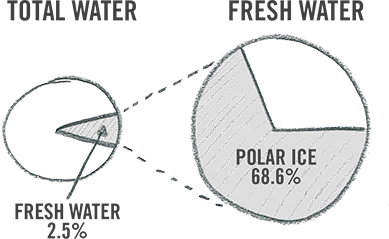
Living systems need clean water to survive, but as you can see, even though there's water all around us, we can only use a small portion of it. That's why we need to manage the small percentage of water that is available to us in a drinkable, potable form.
A water molecule is made up of one oxygen atom bonded to two hydrogen atoms in such a way that it can stick together or stick to other things. This unusual structure makes water very useful.
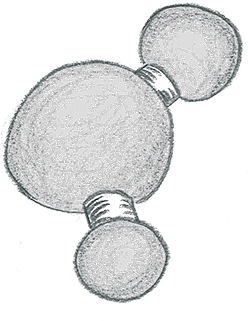
For example, in a water molecule, the oxygen atom has a slightly negative electrical charge and the hydrogen atoms have a slightly positive electrical charge which allows them to not only attract each other, but also to attract atoms from other water molecules too. That means they can work together to hold many water molecules together. A water molecule's electrical charge also attracts molecules of other substances, which is what makes those substances dissolve in water. In fact, more substances dissolve in water than in any other solvent, so it is called a universal solvent.

Water is also unique in that it is able to exist naturally in all three states of matter: as solid ice and snow, as liquid water, and as a gas in the atmosphere. Water changes between these three states when cooled or heated:
• Water freezes at 0° Celsius, and as it freezes molecules move apart to form crystals which makes the water expand by about 10 percent. That's why ice is less dense than water, and is why it floats.
• Water is the least dense at -4° C and most dense at 0° C.
• If we heat a volume of water above 100° Celsius, the water molecules begin to move far apart and at high speeds. As a result, the molecular forces become weaker and the water changes into a gas called water vapour.
Water quality describes how pure or clean the water is and is determined by the kinds and amounts of substances dissolved and suspended in the water. However, the standards for water quality are different depending on how the water will be used. Drinking water, for example, is regulated by guidelines strict enough to protect human health, because a lack of such guidelines could lead to a variety of health problems.
But we know that just looking at water in a glass won't tell us if the water is safe to drink. So to determine the quality of the water we need to run a variety of tests. There are many different kinds of tests that can be run, so we'll focus on a few of the most common. All of these tests involve collecting water, running tests, and comparing the results to a set of standards.

Salinity
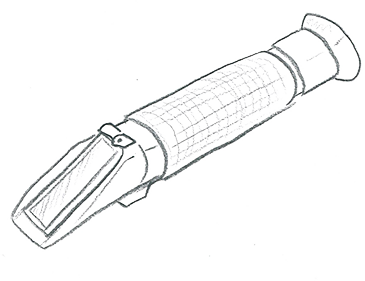
The most common substances found dissolved in water are salts. The total amount of all salts found in water is called its salinity. Adding salt to the water creates what's known as soft water, while water described as hard is high in dissolved minerals, specifically calcium and magnesium.
To measure salinity, we use a refractometer. It works using the refractive index, which changes based on the amount of dissolved salt in the water.
Turbidity
Turbidity is a measure of the clarity of water, or how many particulates (tiny particles of stuff) are floating around in it. When water is clear, we say the water has low turbidity. So, if you can see to the bottom of a pond on a warm summer day, the water has low turbidity. If, on the other hand, the water is murky, we say it has high turbidity. So if you looked at that same pond after a rainstorm when all the muck has been stirred up, you won't be able to see the bottom because the water has high turbidity.
Scientists measure turbidity by using Secchi Disks, which consist of a weighted plate attached to a rope that has been marked at measured intervals. Secchi depths are measured by slowly lowering the disk into the water and averaging the depths at which the disk disappears and then reappears as it is raised. In the real world, scientists use turbidity measurements to calculate inputs from erosion and nutrients.
Toxic substances and other pollutants
Scientists also test for many harmful things in water like metal, pesticides, and oil. For example, scientists are finding mercury, which can come from mining or air pollution in addition to natural sources, in certain types of fish, especially in lakes and estuaries.
Finding these harmful substances means using analytical methods to determine the concentration of a chemical compound or chemical element. There are a wide variety of techniques used for analysis including spectroscopy, chromatography and titration.
Bacteria
Scientists also sample for certain types of bacteria that are found only in the intestinal tract of animals and humans. These bacteria, called fecal coliforms, are not necessarily harmful, but they're usually found with some bad characters like viruses and pathogens, which can make you sick. The major sources of fecal coliforms are failing septic systems, wastewater treatment plant discharges, and animal waste.
One example of a type of bacteria you've probably heard of is E. coli. Scientists test for E. coli using an indicator made of nutrients that cause target microbes contained in the sample to change to confirm the presence of E. coli.

pH
pH is measured to determine the concentration of hydrogen ions in the water, which affects how acidic or basic the water is. pH (which stands of "potential of Hydrogen") ranges from 0 (very acidic) to 14 (very basic), with 7 being neutral and most waters ranging from 6.5 to 8.5. Changes in pH can affect how chemicals dissolve in the water and can be harmful to living things.
For example, high or low acidity can be deadly to fish and other aquatic organisms. pH is measured by using indicators or devices such as a pH pen.
Every industry in the world uses water in at least some of their processes. From steam assisted gravity drainage used to recover bitumen, to water floods used to develop mature conventional fields, to water based extraction of oil sands, you'll find water used everywhere!
As a result, these industries need to follow guidelines to ensure they aren't changing the quality of water or using too much water. That means each company that uses water is responsible for ensuring water quality is maintained at safe levels. The goal is always to return the water at the same quality in which it was taken.
Water quality must meet acceptable levels to be considered safe, so guidelines have been set up to protect water. Depending on how the water is being used, different guidelines are used to determine if the water is safe.
• The Canadian Drinking Water Guidelines outlines levels that are safe for the water that we consume.
• The Canadian Recreational Water Quality Guidelines are used to test water used for recreation.
• Industries need to follow a variety of guidelines, like the Alberta River Water Quality Index, to make sure the water they return to the environment is safe.
Ultimately, water quality needs to be managed constantly to ensure it is safe for us and all the organisms in the environment.
- Home
-
Help
How to Use
This is your chance to explore water and water quality, and there are four sections to help you do it..
The Basics
Want to know more information about water? Head to the basics section and get the scoop on water.
Experiment
Here is your chance to experiment and test some samples for yourself. Check your water sample against some water quality guidelines and see if they make the cut.
Challenge
Now it's time to apply all your knowledge. Complete the challenge and see how you stack up.
Test Yourself
Think you know it all? Test yourself to see if you are the real deal! You have three questions to answer, but if you're feeling brave you can add some more.
If you're ever unsure of what a word means, check out the definition in the glossary! You can also click on any highlighted word to take you straight there.
-
Teacher Info
Overview
Water Quality provides students an opportunity to explore a science topic in their own way, at their own pace.
The four sections of the tool have been designed to guide students through the process of understanding water quality and let them put that information into practice. Students will gain knowledge of the topic by reading the basics, experimenting without consequence to learn the techniques, applying their knowledge to solve a challenge, and assessing their knowledge with a self-test. A comprehensive glossary also lets them check definitions at any time along the way.
This tool provides a way to teach the elements of the science unit, but also puts science into perspective with real world examples, challenges students to use technology to further their understanding of science, and engages them with a wide range of activities.Curriculum
Grade 8 – Fresh and Salt Water Systems
SLE #1 – Describe the distribution and characteristics of water in local and global environments, and identify the significance of water supply and quality to the needs of humans and other living things
SLE #4 – Analyze human impacts on aquatic systems; and identify the roles of science and technology in addressing related questions, problems and issues.
-
Glossary
- Concentration
- The amount of a solute, chemical, or pollutant in a specified amount of solvent, air, water, soil or other medium.
- Contaminant
- A substance that causes harm and renders water unfit for its intended use.
- Freshwater
- Water that has a much lower solute concentrations than the ocean, or water containing less than 1,000 parts per million (ppm) of dissolved solids of any type.
- Parts per Million (ppm)
- The number of "parts" by weight of a substance per million parts of water. This unit is commonly used to represent pollutant concentrations. One milligram per liter of water is approximately equal to a ppm.
- Potable
- Water that is suitable for drinking.
- Salinity
- A quantitative measure of the amount of dissolved salts in a given volume of water.
- Solute
- Any solid material that is dissolved in a liquid (the solvent).
- Solvent
- The liquid, usually water, in which solutes are dissolved; a substance that dissolves other substances, thus forming a solution.
- Turbidity
- A measure of cloudiness or opaqueness in the water. Turbidity is measured in nephelometric turbidity units (NTU).
- Water Quality
- The chemical, physical, and biological characteristics of water, usually in respect to its suitability for a particular purpose.
- Credits
Your overall score
Read each slide to earn maximum points.
Test all five water samples to earn maximum points.
Identify water quality at all five locations to earn maximum points.
Earn 5 points for every question correctly answered.
